Aligning Health, Elevating Life: Your Journey to Wellness Begins Here!
A holistic approach designed to unlock your body's innate healing potential and promote total well-being. At the core of our philosophy is the belief that true health involves more than just the absence of pain; it encompasses the balance of your physical, mental, and emotional aspects.
Committed to Your Health and Wellness Journey
Welcome to our website, where our passion for health and commitment to holistic well-being converge. Founded with a vision to redefine healthcare, we take pride in being more than just a chiropractic clinic—we are partners in your journey towards optimal health.
Recognizing that each person is unique, we prioritize a patient-centered approach. From the moment you walk through our doors, our goal is to understand your individual needs, concerns, and goals.
15
Years of Experience
Our Vision
We envision a world where individuals are empowered to take control of their health,

Our Mission
We aim to be a trusted partner in our patients' pursuit of a vibrant and pain-free life.
Committed to Your Health and Wellness Journey
Welcome to American Chiropractic Medical Services, where our passion for health and commitment to holistic well-being converge. Founded with a vision to redefine healthcare, we take pride in being more than just a chiropractic clinic—we are partners in your journey towards optimal health.
Recognizing that each person is unique, we prioritize a patient-centered approach. From the moment you walk through our doors, our goal is to understand your individual needs, concerns, and goals.
15
Years of Experience

Our Vision
We envision a world where individuals are empowered to take control of their health,

Our Mission
We aim to be a trusted partner in our patients' pursuit of a vibrant and pain-free life.
Conditions Under Our Expertise
Chiropractic Care
Unleashing natural healing through spinal alignment and holistic well-being.
Acupuncture
Balancing energy flow for overall health and wellness.
Massage Therapy
Relieving tension and promoting relaxation for a body and mind.
Sports Chiropractic
Optimizing performance and preventing injuries for athletes of all levels.
Laser Therapy
Harnessing the power of light for targeted pain relief and healing.
Pediatric Chiropractic
Nurturing the health and well-being of children through gentle care.
1,200 +
Happy Patients
15 yr
Years of Experience
250 +
Therapist & Staff
75 +
Branch Clinic
Our Patient Reviews
Choosing you was the best decision for my health. The personalized care, expert guidance, and transformative approach made a significant impact on my well-being.
I am grateful for the dedicated team who not only alleviated my pain but empowered me with the knowledge to sustain a healthier lifestyle. Trustworthy, compassionate, and effective – I highly recommend you for anyone seeking holistic chiropractic care."
Jane Doe
Get In Touch
+17703299784
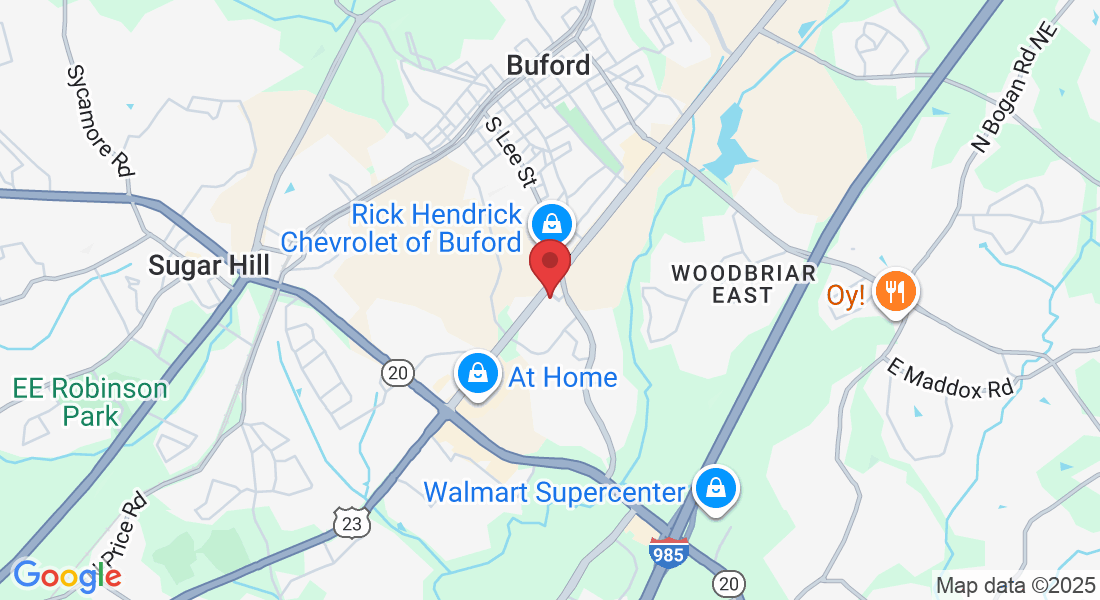
Address: 1879 Buford Highway, suite 4, Buford GA 30518
Email: support@yourchiropractichealth.com
Assistance Hours:
Mon – Sat 8am to 6pm
Sunday – Closed
"Super friendly staff, great doctor, will definitely be back." - Amy